PRODUCT IDENTIFICATION
H.S. CODE
TOXICITY
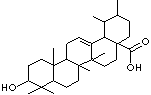
C1[C@@]2([C@@H]3[C@@](CC[C@H]2C(C)([C@H](C1)O)C)([C@@]1(C(= CC3) [C@H]2[C@@](CC1)(CC[C@H]([C@@H]2C)C)C(O) =O)C)C)C
CLASSIFICATION
Triterpenoid, Analgesic, Anti-Inflammatory, Anti-infective, Cyclooxygenase Inhibitor
EXTRA NOTES
PHYSICAL AND CHEMICAL PROPERTIES
AUTOIGNITION
NFPA RATINGS
REFRACTIVE INDEX
EXTERNAL LINKS & GENERAL DESCRIPTION
Wikipedia Linking - Ursolic acid
Google Scholar Search - Ursolic acid
Drug Information Portal (U.S. National Library of Medicine) - Ursolic acid
PubChem Compound Summary - Ursolic acid
KEGG (Kyoto Encyclopedia of Genes and Genomes) - Ursolic acid
http://www.ebi.ac.uk/ - Ursolic acid
http://www.ncbi.nlm.nih.gov/ - Ursolic acid
http://toxnet.nlm.nih.gov/
Hazardous Substances Data Bank - Ursolic acid
http://netchina.net/
Ursolic acid, a carboxylic acid present in a wide
variety of plants in the form of a free acid or an aglycone of triterpene
saponins.It is also known as urson, prunol, micromerol, and malol, is a
pentacyclic triterpenoid compound which naturally occurs in a large number of
vegetarian foods, medicinal herbs, and plants,. For a long time, it was
considered to be pharmacologically inactive. Thus, ursolic acid and its alkali
salts (e.g. potassium or sodium ursolates) were exclusively used as emulsifying
agents in pharmaceutical, cosmetic, and food preparations3,4. However, upon
closer examination, ursolic acid was found to be medicinally active both
topically and internally1. Its anti-inflammatory, antitumor (skin cancer), and
antimicrobial properties make it useful in cosmetic applications1. Ursolic
acid [(3b)-3-Hydroxyurs-12-en-28-oic acid] rarely occurs without its isomer
oleanolic acid [(3b)-3-Hydroxyolean-12-en-28-oic acid]. They may occur in their
free acid form, or as aglycones for triterpenoid saponins
which are comprised of a triterpenoid aglycone linked to one or more sugar
moieties. Ursolic and oleanolic acids are similar in pharmacological activity.
Local
Ursolic acid is a pentacyclic triterpenoid compound present with its isomer
oleanolic acid in many plant kingdom. They may occur as aglycones of glycoside
molecules or in their free form. Ursolic and oleanolic acids are known to have
pharmacological effect for skin cancer therapy. They, as phytochemicals, have
some potential pharmacological activities including antiinflammatory,
antimicrobial, antitumor, antioxidant and antiviral activity. Ursolic acid, or
derivatives thereof, is used as the active ingredient in many cosmetic
preparations.
APPEARANCE
95.0% min
LOSS ON DRYING
0.5% max
HEAVY METALS
10ppm max
HAZARD OVERVIEW
May be harmful if inhaled. May cause respiratory tract irritation. May be harmful if absorbed through skin. May cause skin irritation. May cause eye irritation. May be harmful if swallowed. OSHA Hazards:Not known
RISK PHRASES
SAFETY PHRASES
24/25